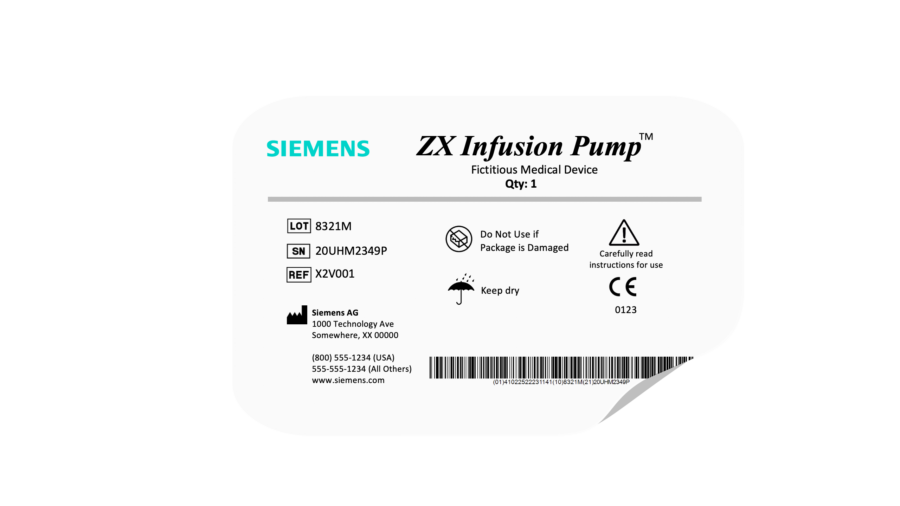
Label The Device . this guidance document describes the general labeling principles for medical devices and ivd medical devices and. understanding each section of a typical device label, and why it’s needed, will help ensure the labeling process is as efficient as. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,.
from blogs.sw.siemens.com
understanding each section of a typical device label, and why it’s needed, will help ensure the labeling process is as efficient as. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. this guidance document describes the general labeling principles for medical devices and ivd medical devices and. learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on.
Siemens PLM for Medical Devices Labeling and UDI solution
Label The Device this guidance document describes the general labeling principles for medical devices and ivd medical devices and. (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. understanding each section of a typical device label, and why it’s needed, will help ensure the labeling process is as efficient as. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. this guidance document describes the general labeling principles for medical devices and ivd medical devices and. learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on.
From hiveta.com
Label Compliance AB&R® (American Barcode and RFID) Label The Device (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. this post will discuss what counts as a medical device label, where they are. Label The Device.
From exodjaqsq.blob.core.windows.net
Tga Medical Device Labeling Requirements at Tyrone Gaylord blog Label The Device this guidance document describes the general labeling principles for medical devices and ivd medical devices and. learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. this post will discuss what counts as a medical device label, where they are required, and look at the. Label The Device.
From autoctrls.com
How to Properly Label Devices in a Circuit Diagram Label The Device this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. understanding each section of a typical device label, and why it’s needed, will help ensure the labeling process is as efficient as. (a) the label of a device in package. Label The Device.
From coastlabel.com
Medical Device Labeling Medical Equipment Labels Coast Label Label The Device (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. understanding each section of a typical device label, and why it’s needed, will help ensure the labeling process is as efficient as. this post will discuss what counts as a medical device label, where they are. Label The Device.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Label The Device learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. this post will discuss what counts as a medical device label, where they are. Label The Device.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Label The Device (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. this guidance document describes the general labeling principles for medical devices and ivd medical devices and. this post will discuss what counts as a medical device label, where they are required, and look at the key. Label The Device.
From mavink.com
Medical Device Labeling Symbols Label The Device this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. understanding each section of a typical device label, and why it’s needed, will help ensure the labeling process is as efficient as. (a) the label of a device in package. Label The Device.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Label The Device (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. understanding each section of a typical device label, and why it’s needed, will help. Label The Device.
From www.regdesk.co
HSA Guidance on Labeling for Medical Devices Introduction RegDesk Label The Device learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. understanding each section of a typical device label, and why it’s needed, will help. Label The Device.
From tramexmeters.com
Datalogging with Tramex Meters App Label The Device learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. (a) the label of a device in package. Label The Device.
From guidewiringthorsten88.z19.web.core.windows.net
Label The Devices In The Circuit Diagram Label The Device (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. understanding each section of a typical device label, and why it’s needed, will help ensure the labeling process is as efficient as. this post will discuss what counts as a medical device label, where they are. Label The Device.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Label The Device understanding each section of a typical device label, and why it’s needed, will help ensure the labeling process is as efficient as. (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. this post will discuss what counts as a medical device label, where they are. Label The Device.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Label The Device learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. this guidance document describes the general labeling principles. Label The Device.
From www.kmedhealth.com
Medical Device Label SymbolsI In Packaging Label The Device (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. understanding each section of a typical device label, and. Label The Device.
From mavink.com
Medical Device Labeling Symbols Label The Device this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. (a) the label of a device in package. Label The Device.
From texaslabelprinters.com
Medical Device Label Printers UDI Label Printers Label The Device this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. this guidance document describes the general labeling principles. Label The Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Label The Device (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. learn about the labeling requirements for medical devices, including regulations, types of labels required, and the information that must be included on. this post will discuss what counts as a medical device label, where they are. Label The Device.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Label The Device this post will discuss what counts as a medical device label, where they are required, and look at the key points of us and eu medical device. (a) the label of a device in package form shall specify conspicuously the name and place of business of the manufacturer,. understanding each section of a typical device label, and. Label The Device.